Wearable medical sensors that help patients and clinicians monitor health conditions are transforming the way medical care is administered. Use of these cutting-edge devices is a fast-growing trend, and the market is starting to boom. At Eastprint, an East West Business, we stay ahead of the curve in this emerging field to help our clients leverage market opportunities for consumer healthcare wearables.
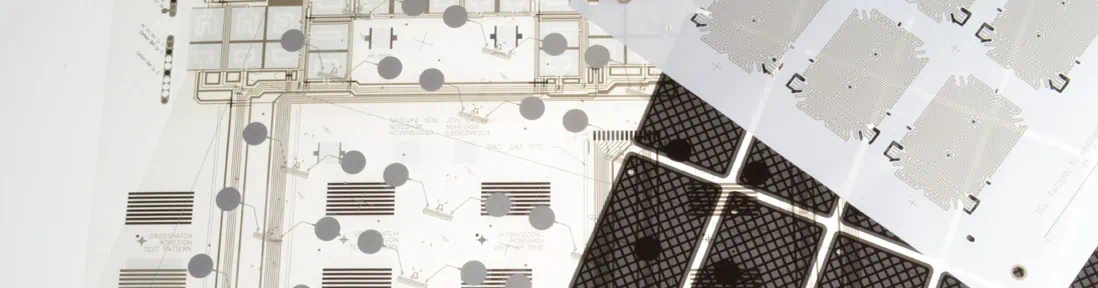
With the scope of our capabilities ranging from product development to full-scale FDA contract manufacturing, we have the technical expertise to address the complexity and rigor associated with introducing new consumer healthcare products to the market. One example is the disposable seizure monitoring sensor highlighted here. Our profound knowledge of silver/silver chloride inks and hydrogel sensing materials, combined with our comprehensive manufacturing and quality assurance capabilities, allowed us to make a significant contribution towards the successful product launch.
We overcame many challenges related to the design, engineering, and manufacturing of the components for this wearable device. From thermoforming out-of-the-ordinary soft-touch materials for the protective cover to double-sided through-hole printing to allow connectivity on both sides of the sensor substrate, and close tolerance layer-to-layer assembly, we engineered innovative, ISO and FDA-compliant processes to keep quality high and costs low.
After several months of prototyping, alpha testing, beta testing, and clinical trials, the product was approved for consumer use. We were awarded the full production contract for this device. As part of our end-to-end services, we also implemented a custom packaging solution that involved manufacturing a foil lined sealed pouch for packaging the individual sensors. With the cost-sensitive nature of this product, we manufacture it in our Mexico facility in the range of 25,000 to 100,000 units per order.
With expert engineering skills representing all relevant disciplines, we can help you address the complex challenges of wearable medical device development. Contact us today to discuss your latest idea.
Disposable Seizure Monitoring Sensor Project Highlights
- Project Description
Disposable Seizure Monitoring Sensor
- Capabilities Applied/Processes
Primary:
CNC Drill and Rout
Screen Printing
Thermoforming
Die Cutting
Injection Molding
Secondary:
Glue Attach of Plastic Cradle
Mechanical Eyelet Attach of Lithium Coin Cell Battery
Layer Laminations
Medical Hydrogel Installation
Heat Seal Protective Individual Pouching and Labeling
FDA Registration
- Equipment Used to Manufacture Part
Flatbed Screen Printing Press
Horizontal Heated Platen Forming Press
CNC Drilling Equipment
Pneumatic Dispense and UV Curing System
Pneumatic Eyelet Insertion Equipment
Clamshell Die-Cutter
Custom Assembly Fixtures
Heat Sealing Equipment
- Overall Part Dimensions
2.087″ x 5.346″
- Tightest Tolerances
+/-.010″
- Material Used
Various: Polyester, ABS, Closed Cell Polyethylene foam, Medical Grade Pressure sensitive skin adhesives, Conductive Silver and Silver/Silver Chloride, Hydrogel Sensing Gel, Lithium Coin Cell battery, Foil lined sealed pouch material. Miniature Conductive eyelets.
- Material Finish
Soft Touch Closed Cell White Polyethylene
- Industry for Use
Consumer Home-based Medical Monitoring Sensor
- In Process Testing/Inspection Performed
Electrical Functional Open – Shorts, Battery Voltage Verification, Bond Strength, pouch burst testing for verification of seal integrity.
- Delivery Location
United States
- Standards Met
- ISO 13485
FDA Compliant
IQ/OQ/PQ - RoHS
Conflict Minerals
REACH
- ISO 13485